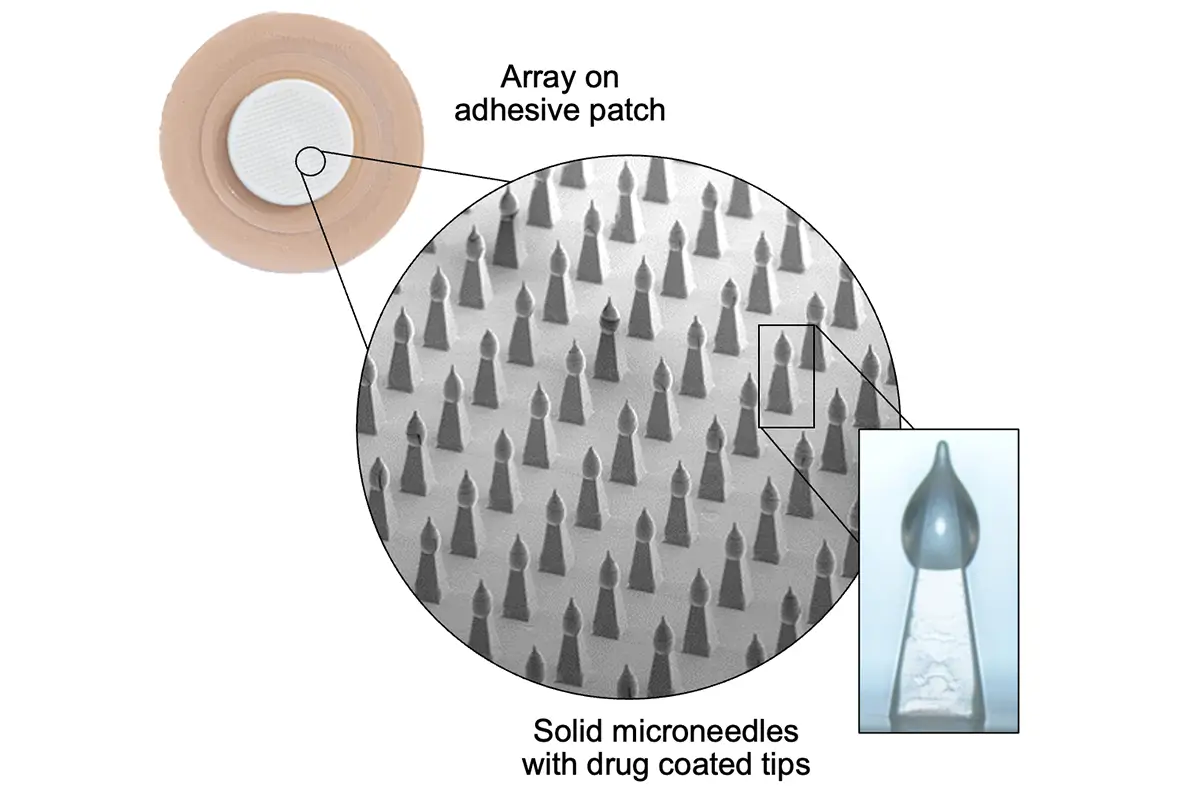
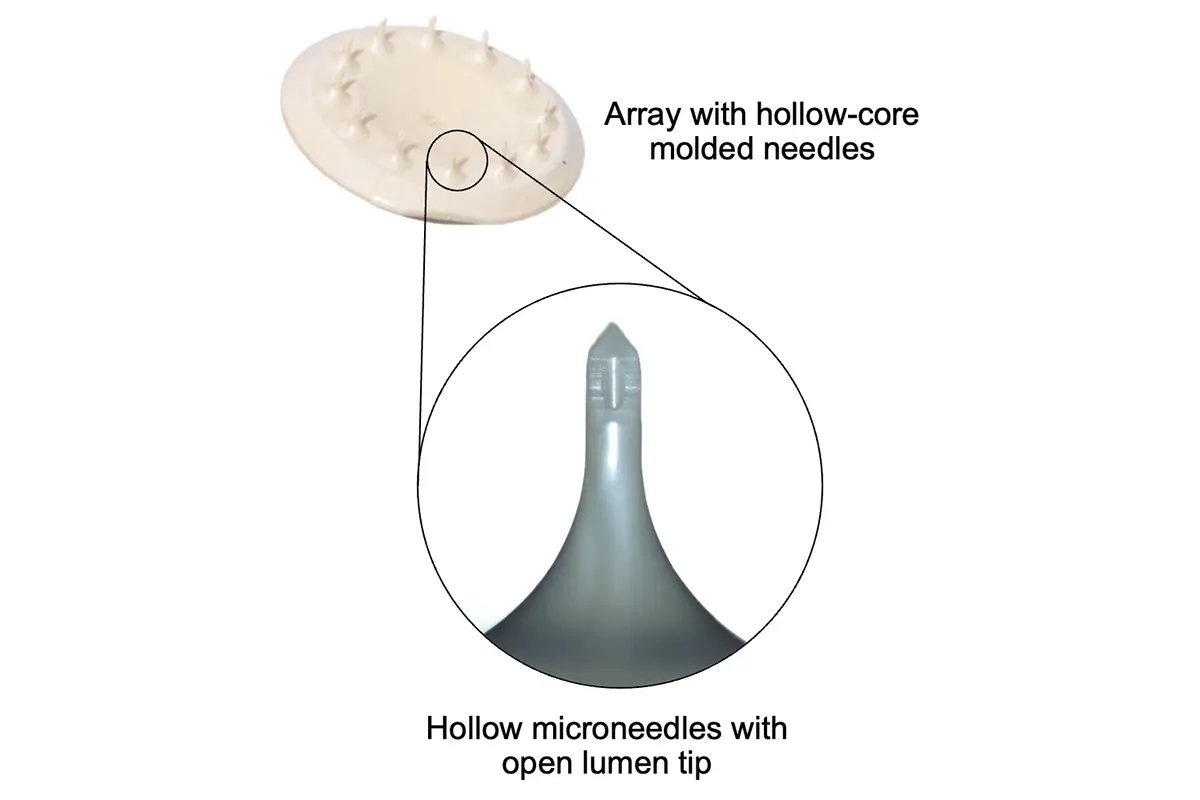
Our microneedle array patches are designed for accurate and reliable intradermal delivery of small and large molecules including vaccines, peptides, proteins, and biologics. Our unparalleled capabilities and expertise deliver an exceptional product customized for your patients and brand, and we are ready to supply lab, clinical, and commercial volumes.
The potential benefits of microneedle drug delivery
Microneedle array patches have the potential to deliver benefits across the medical system, patients and their caregivers, and pharmaceutical manufacturers through innovative intradermal delivery.
Within the medical system, this could offer:
- Greater efficacy through PK improvement and enhanced immunogenicity
- Faster onset of action
- Better patient compliance/adherence
- Elimination of cold-chain storage, improving vaccine access for hard-to-reach populations
For patients and caregivers, this could provide:
- Preferred administration experience
- At-home dosing vs. clinic/hospital
- Ease of use
- Convenience
- Reduced impact of needle phobia
For pharmaceutical manufacturers, this could result in:
- Dose-sparing
- Market expansion through self-administration
- Reduced COGs and distribution costs
- Life cycle extension
- Differentiation by ROA and by device/product
Our manufacturing capabilities
Your specialized drug delivery can be realized through our extensive capacity and unparalleled capability; for solid microneedle delivery, we can aseptically manufacture >1 million patches per year for clinical trials and have commercial manufacturing capabilities to produce ~14 million patches per year.
Microneedle array patch design considerations
Through extensive lab & clinical testing, we have identified significant contributors to drug delivery efficacy and are continuously expanding our datasets around these features. Work with the expert team that understands the interrelationships of these variables and can help you to create an optimal design for your target product profile:
- Space between arrays
- Microneedle Length
- Droplet volume/solids per microneedle
- Droplet shape
- Coated Formulations
- Number of microneedles
Bring your product to market
Our team has the expertise necessary to support the entire life span of your microneedle array patch product, from early-phase development through launch and post-commercialization. Every step of the way, we remain committed to upholding the highest quality standards, ensuring reliable and safe delivery for every patient, every time.
With complex and ever-evolving regulations governing microneedle drug delivery technology, you need an experienced CDMO partner. Kindeva is leading the way in this space and we stay up to date with the latest guidelines and best practices.